Intro
Discover the comprehensive Polyatomic Ions List, featuring common ions like ammonium, nitrate, and sulfate, along with their charges and chemical formulas, to master chemistry concepts like molecular structure and ionic bonding.
The world of chemistry is vast and complex, with numerous concepts that help us understand the behavior of elements and compounds. One such concept is polyatomic ions, which are groups of atoms that carry a charge. Understanding polyatomic ions is essential for grasping various chemical reactions and processes. In this article, we will delve into the world of polyatomic ions, exploring their importance, types, and applications.
Polyatomic ions are crucial in chemistry because they help explain how compounds form and react. They are composed of multiple atoms that are chemically bonded together and carry a net charge, either positive or negative. This charge is what makes polyatomic ions so important, as it allows them to participate in chemical reactions and form new compounds. The study of polyatomic ions is vital for understanding various chemical processes, including acid-base reactions, precipitation reactions, and oxidation-reduction reactions.
The importance of polyatomic ions cannot be overstated. They are used in various industries, including pharmaceuticals, agriculture, and manufacturing. For instance, polyatomic ions are used in the production of fertilizers, which are essential for plant growth and agriculture. They are also used in the manufacture of certain medications, which rely on the unique properties of polyatomic ions to function effectively. Furthermore, polyatomic ions play a critical role in environmental science, as they help us understand and mitigate the effects of pollution on our ecosystem.
Introduction to Polyatomic Ions
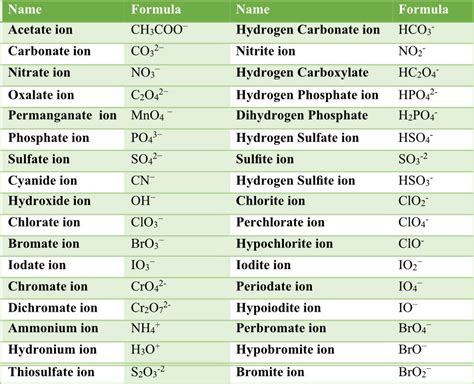
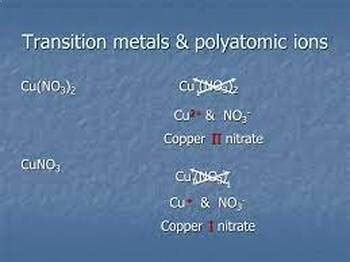
Polyatomic ions are typically composed of a central atom surrounded by one or more other atoms. The central atom is usually a metal, while the surrounding atoms are nonmetals. The charge on a polyatomic ion is determined by the number of electrons gained or lost by the central atom. For example, the ammonium ion (NH4+) has a positive charge because the nitrogen atom has gained four electrons from the surrounding hydrogen atoms.
Types of Polyatomic Ions
There are several types of polyatomic ions, each with its unique properties and characteristics. Some common types of polyatomic ions include: * Cations: These are polyatomic ions with a positive charge. Examples include the ammonium ion (NH4+) and the hydronium ion (H3O+). * Anions: These are polyatomic ions with a negative charge. Examples include the carbonate ion (CO32-) and the sulfate ion (SO42-). * Oxides: These are polyatomic ions that contain oxygen. Examples include the nitrate ion (NO3-) and the phosphate ion (PO43-).Common Polyatomic Ions
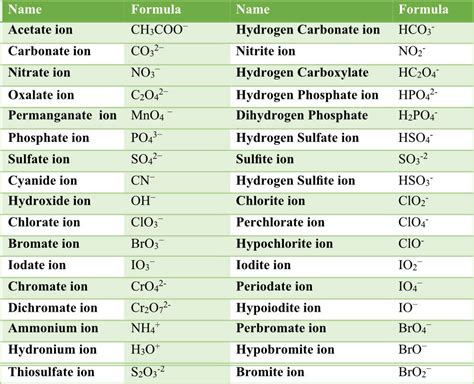
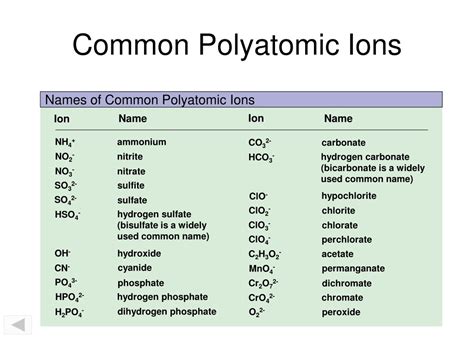
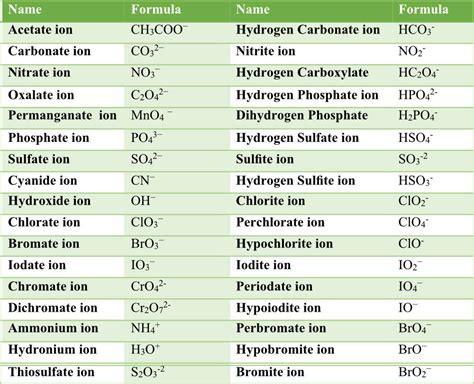
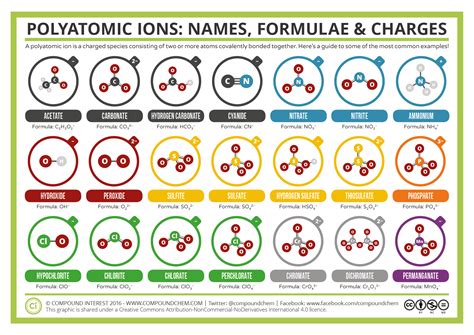
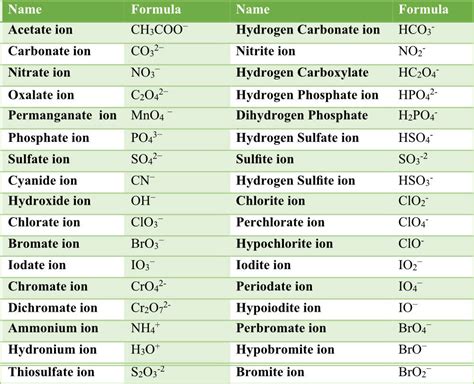
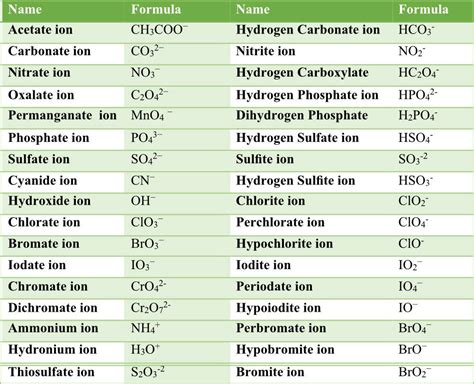
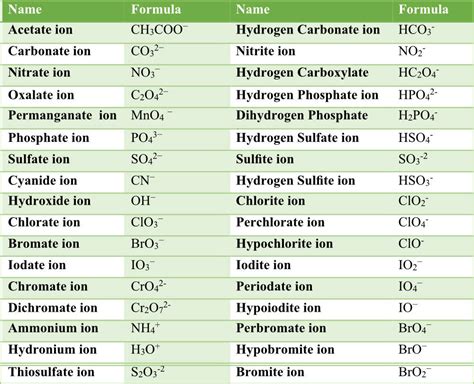
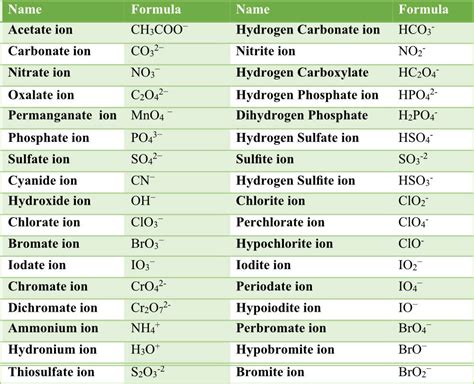
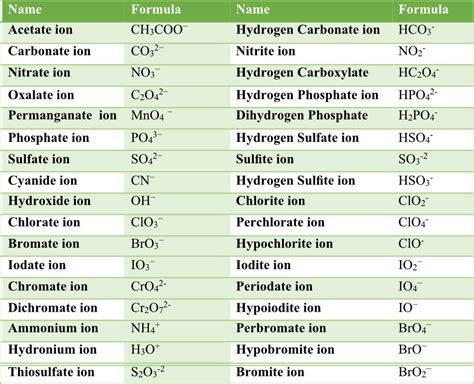
Some common polyatomic ions include:
- Ammonium (NH4+)
- Carbonate (CO32-)
- Nitrate (NO3-)
- Phosphate (PO43-)
- Sulfate (SO42-)
- Chlorate (ClO3-)
- Perchlorate (ClO4-)
- Bromate (BrO3-)
- Iodate (IO3-)
- Hydroxide (OH-)
These polyatomic ions are commonly found in nature and are used in various industrial and commercial applications. For example, the ammonium ion is used in the production of fertilizers, while the carbonate ion is used in the manufacture of glass and ceramics.
Properties of Polyatomic Ions
Polyatomic ions have several unique properties that make them useful in various applications. Some of these properties include: * Charge: Polyatomic ions have a net charge, which allows them to participate in chemical reactions. * Size: Polyatomic ions can vary in size, depending on the number of atoms they contain. * Shape: Polyatomic ions can have different shapes, depending on the arrangement of their atoms. * Solubility: Polyatomic ions can be soluble or insoluble in water, depending on their properties.Applications of Polyatomic Ions
Polyatomic ions have numerous applications in various fields, including:
- Pharmaceuticals: Polyatomic ions are used in the production of certain medications, such as antacids and anti-inflammatory drugs.
- Agriculture: Polyatomic ions are used in the production of fertilizers, which are essential for plant growth and agriculture.
- Manufacturing: Polyatomic ions are used in the manufacture of glass, ceramics, and other materials.
- Environmental science: Polyatomic ions help us understand and mitigate the effects of pollution on our ecosystem.
Importance of Polyatomic Ions in Chemistry
Polyatomic ions are essential in chemistry because they help explain how compounds form and react. They are used to: * Predict the properties of compounds * Understand chemical reactions * Identify the composition of compounds * Develop new materials and productsIn conclusion, polyatomic ions are a crucial concept in chemistry, with numerous applications in various fields. Understanding polyatomic ions is essential for grasping various chemical processes and reactions. By recognizing the importance of polyatomic ions, we can appreciate their role in shaping our world and improving our lives.
Gallery of Polyatomic Ions
Polyatomic Ions Image Gallery
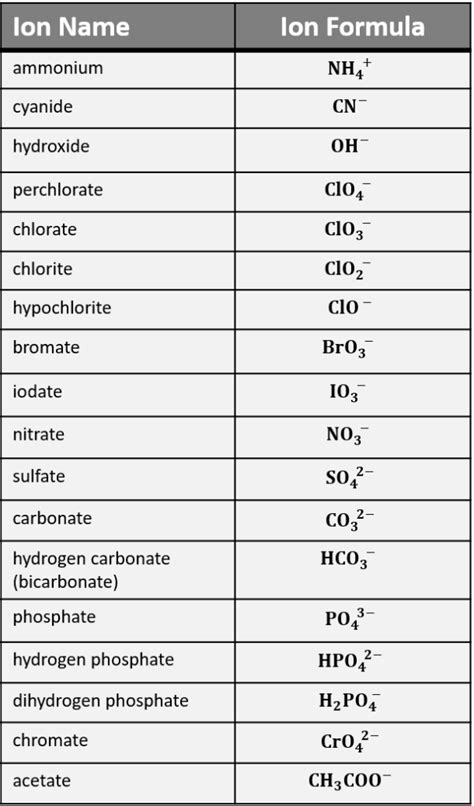
What are polyatomic ions?
+Polyatomic ions are groups of atoms that carry a charge. They are composed of multiple atoms that are chemically bonded together and carry a net charge, either positive or negative.
What are the types of polyatomic ions?
+There are several types of polyatomic ions, including cations, anions, oxides, and others. Each type has its unique properties and characteristics.
What are the applications of polyatomic ions?
+Polyatomic ions have numerous applications in various fields, including pharmaceuticals, agriculture, manufacturing, and environmental science. They are used in the production of fertilizers, medications, glass, ceramics, and other materials.
We hope this article has provided you with a comprehensive understanding of polyatomic ions and their importance in chemistry. Whether you are a student, researcher, or simply interested in learning more about chemistry, we encourage you to share this article with others and continue exploring the fascinating world of polyatomic ions. If you have any questions or comments, please feel free to share them below.
