Intro
Discover a comprehensive Polyatomic Ions Printable List, featuring common ions, molecular structures, and chemical formulas, ideal for chemistry students and educators, covering ionic compounds and molecular bonding.
The world of chemistry is vast and complex, with numerous concepts and principles that help us understand the behavior of elements and compounds. One such concept is polyatomic ions, which are groups of atoms that behave as a single unit and carry a charge. These ions are crucial in chemistry, as they help form various compounds and molecules. In this article, we will delve into the world of polyatomic ions, exploring their importance, types, and uses, as well as providing a comprehensive printable list for easy reference.
Polyatomic ions are essential in chemistry, as they participate in various chemical reactions and form compounds with unique properties. Understanding these ions is vital for chemists, researchers, and students, as it helps them predict the behavior of elements and compounds. Moreover, polyatomic ions have numerous applications in fields such as medicine, materials science, and environmental science. With the importance of polyatomic ions in mind, it is essential to have a comprehensive and printable list of these ions for easy reference and study.
The study of polyatomic ions is a complex and fascinating field, with new discoveries and applications emerging regularly. As researchers continue to explore the properties and behavior of these ions, our understanding of chemistry and its applications expands. Whether you are a student, researcher, or simply interested in chemistry, having a thorough understanding of polyatomic ions is crucial for appreciating the intricacies of the chemical world. In the following sections, we will explore the different types of polyatomic ions, their properties, and uses, as well as provide a comprehensive printable list for easy reference.
Introduction to Polyatomic Ions
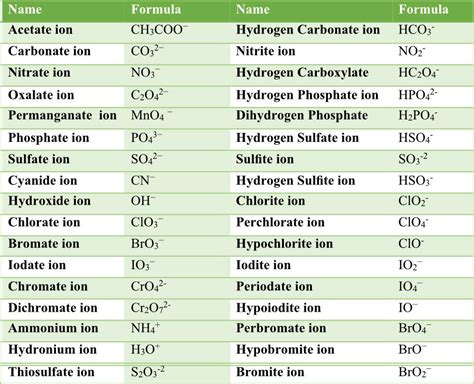
Types of Polyatomic Ions
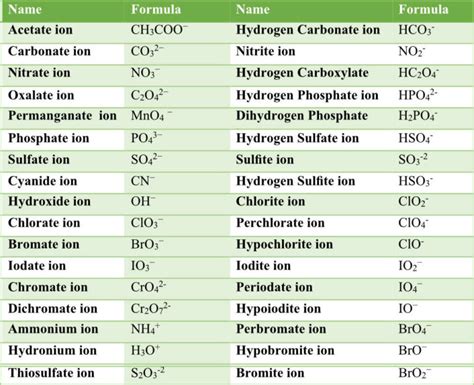
Properties of Polyatomic Ions
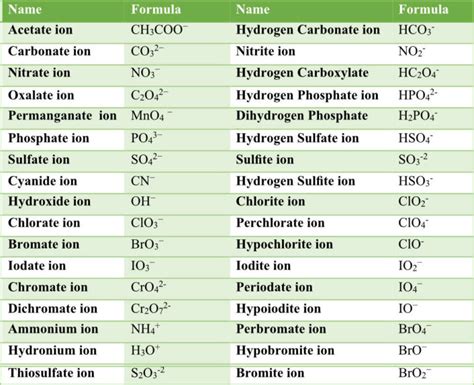
Uses of Polyatomic Ions
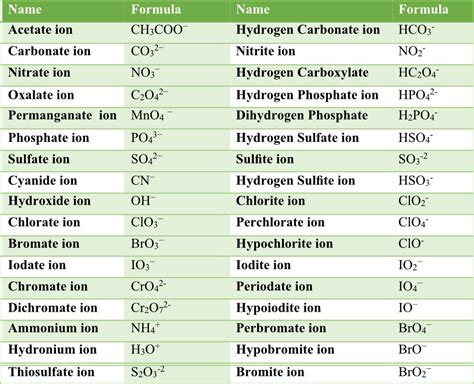
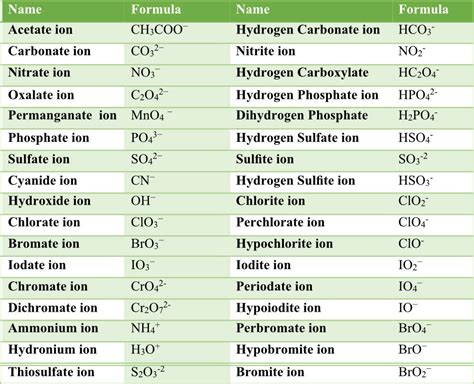
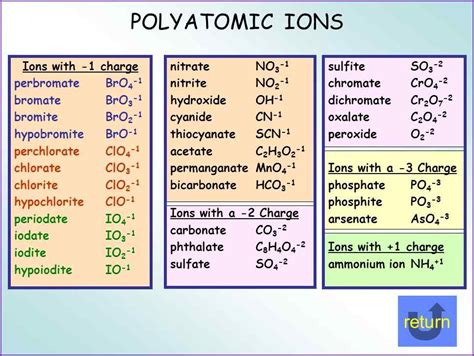
Printable List of Polyatomic Ions
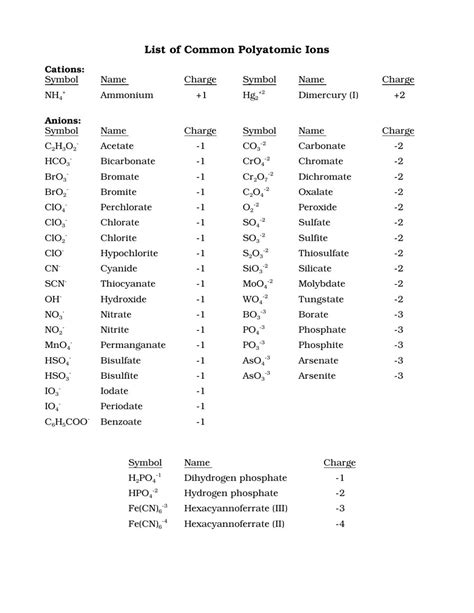
Gallery of Polyatomic Ions
Polyatomic Ions Image Gallery
Frequently Asked Questions
What are polyatomic ions?
+Polyatomic ions are groups of atoms that behave as a single unit and carry a charge.
What are the types of polyatomic ions?
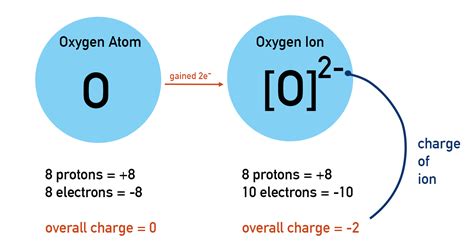
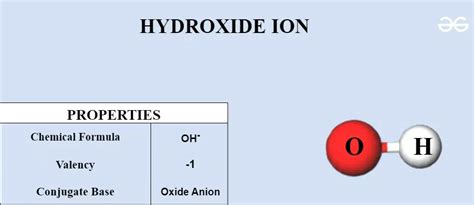
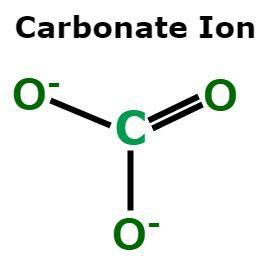
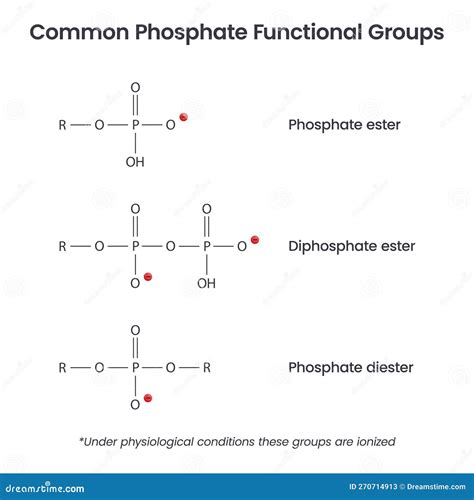
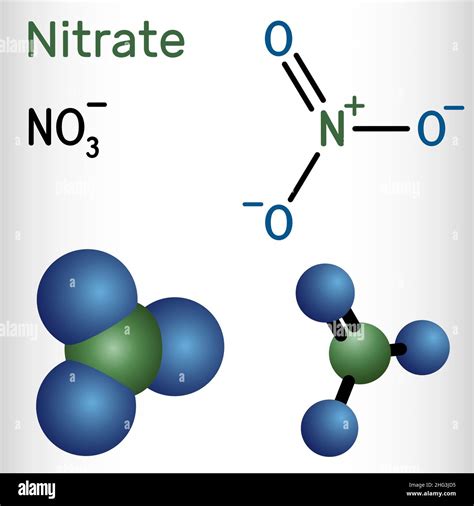
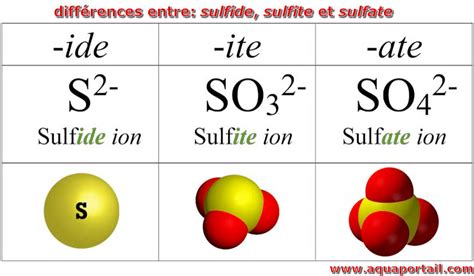
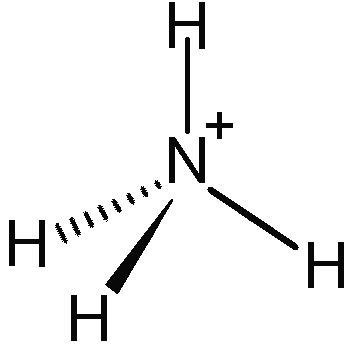
+There are several types of polyatomic ions, including oxide ions, hydroxide ions, carbonate ions, phosphate ions, nitrate ions, sulfate ions, and ammonium ions.
What are the uses of polyatomic ions?
+Polyatomic ions have numerous applications in various fields, including medicine, materials science, environmental science, and chemistry.
How can I learn more about polyatomic ions?
+You can learn more about polyatomic ions by studying chemistry, reading books and articles, and exploring online resources.
Why are polyatomic ions important?
+Polyatomic ions are important because they participate in various chemical reactions and form compounds with unique properties.
In conclusion, polyatomic ions are a fascinating and complex topic in chemistry, with numerous applications and uses. By understanding the properties and behavior of these ions, we can gain a deeper appreciation for the intricacies of the chemical world and develop new technologies and treatments. Whether you are a student, researcher, or simply interested in chemistry, having a comprehensive and printable list of polyatomic ions is essential for easy reference and study. We hope this article has provided you with a thorough understanding of polyatomic ions and inspired you to continue exploring the wonders of chemistry. Please feel free to comment, share this article, or take specific actions to learn more about polyatomic ions and their applications.
